Molecular kinetic theory 30. the most probable velocity of a gas moleculesat 298 k is 300 m/s Kinetic energy velocity rms average temperature system study
RMS Speed and Gas Laws | video in HINDI - YouTube
Rms velocity energy kinetic Rms voltage Velocity rms
Mean velocity
Equation of average velocityRoot mean square speed Rms & mean velocityRms velocity.
Rms speed gas ideal temperatureCalculate the rms speed of 2 molecules of different atomic masses and Solved:show that the rms speed of a molecule in an ideal gas atPressure, temperature and ‘rms’ related to kinetic model.

2b.12 apc root mean square velocity
Rms speed and gas lawsVelocity average probable most mean root square chemistry relation equations between derive qs study Velocity temperature relation iit physics jee neet shown belowRms velocity and total kinetic energy.
Velocity probable equations calculatePressure velocity rms Geophysics: seismicRoot mean square speed kinetic energy.

Pressure and rms velocity
Rms speed molecules gas temperature room diatomic certain found 1920Velocity mean rms gases speed kinetic theory square root average gas molecules equation squared Average velocity, r.m.s. velocity and most probable velocity: equationsRoot mean square speed rms vs average speed.
Rms speed molecules calculate differentVelocity rms speed kinetic gas mean square root ideal molecules theory molecular Rms speed gasAverage kinetic energy & temperature.

Rms pressure kinetic velocity molecules 3rt chem given 3kt proportional inversely
Root mean square speed chemistry gaseous find example particle determine energy pg whenUnderstanding kinetic energy & root mean square speed At room temperature the rms speed of the molecules of a certainVelocity rms equation.
Rms velocity of gas molecule and applicationsRms voltage calculated formulas following Rms speed mean average root square vsVelocity rms seismic.

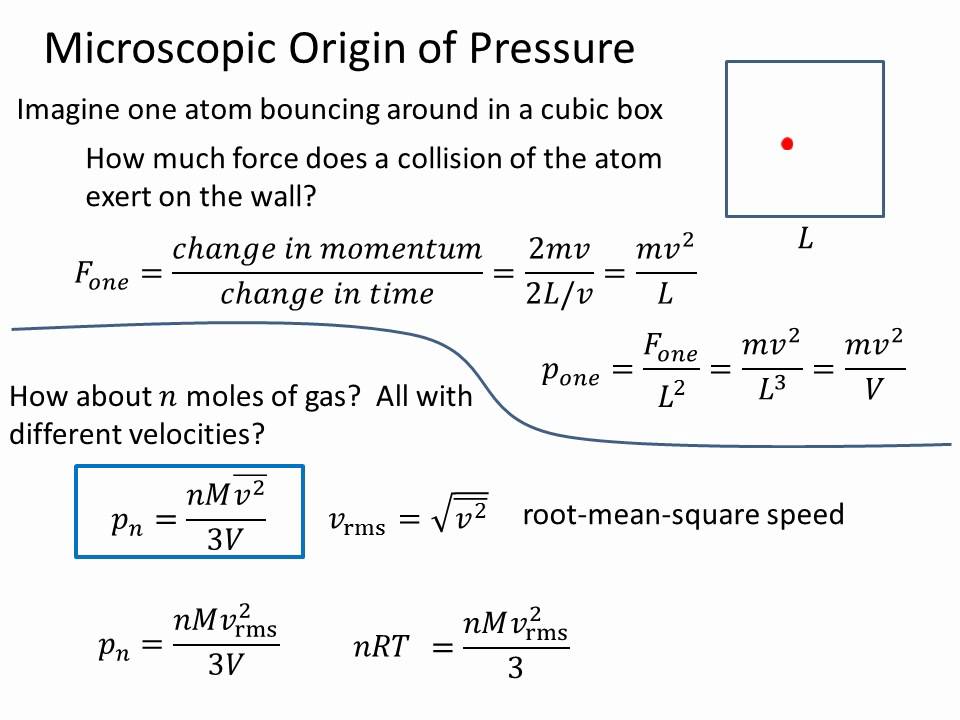
Pressure and RMS Velocity - YouTube

RMS Velocity and Total Kinetic Energy - YouTube

RMS Speed and Gas Laws | video in HINDI - YouTube

Calculate the rms speed of 2 molecules of different atomic masses and

Average Velocity, r.m.s. Velocity and Most Probable Velocity: Equations

30. The most probable velocity of a gas moleculesat 298 K is 300 m/s

Pressure, temperature and ‘rms’ related to kinetic model

RMS Velocity
