1.5-sublevels orbitals and electrons How many orbitals are in the 4p subshell? Nodes orbitals radial 4p quantum socratic spherical increases
1.5-sublevels orbitals and electrons - YouTube
Electrons sublevels energy number sublevel level electron table orbital configuration each many chlorine periodic chart chem does hold chemistry configurations Orbitals chemistry electron atoms subshell order table atomic configurations periodic quantum number structure subshells electronic electrons energies which configuration energy Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answer
Chem orbitals shapes quantum chemistry model atoms theory orbital diagram electrons sublevels sublevel mechanics using wave axes spherical figure shaped
2.2: electron configurationsShapes of orbitals and sublevels Electron configurationsOrbitals 4p subshell diamagnetism orbital electrons valence socratic explain paramagnetism atoms paramagnetic.
Question #d50d3Orbitals subshell 4p orbital degenerate quantum socratic three higher degeneracy Electron configurations orbitals sublevel electrons each has line orbital sublevels chemistry levels hold box within own its theseHow many orbitals are in the 4p subshell?.

Spdf orbitals : parsing spdf orbital hybridization and simple bonding
Electron orbitals energy levels configuration fill configurations orbital order electrons sublevels electronic highest sub filled lowest map filling level increasingOrbitals levels sublevels electron electrons quantum fc2 readingandwritingprojectcom How electrons fill orbitals andOrbitals 2p 1s overlap do chemistry orbital electron quantum following exchange.
Electron orbitals (a-level)Orbitals sublevel shapes sublevels 2s 3s axis identical made Energy levels, sublevels, & orbitalsDo electrons fill the lower energy levels first?.

How many orbitals are in the n = 3 level?
Orbitals electron 2p 3p nucleus atomic 4pEnergy sublevels levels orbitals Orbitals sublevels electrons8.3 development of quantum theory – chem 1114 – introduction to chemistry.
Does the energy of an electron vary in the sublevels?Electron levels electrons orbitals principle aufbau lower sublevel socratic configuration example 4s Filling order of electronsQuantum chemistry.

Filling electrons order shell number maximum chemistry electron configuration each which electronic orbital 4s 3d filled why orbitals sublevels transition
.
.

Do electrons fill the lower energy levels first? | Socratic

quantum chemistry - How do 1s and 2p orbitals overlap? - Chemistry
)/Std_Forms/how-ekect-fill-orbitals-sublevels_files/image006.jpg)
How Electrons fill Orbitals and

How many orbitals are in the 4p subshell? | Socratic
.png)
Question #d50d3 | Socratic
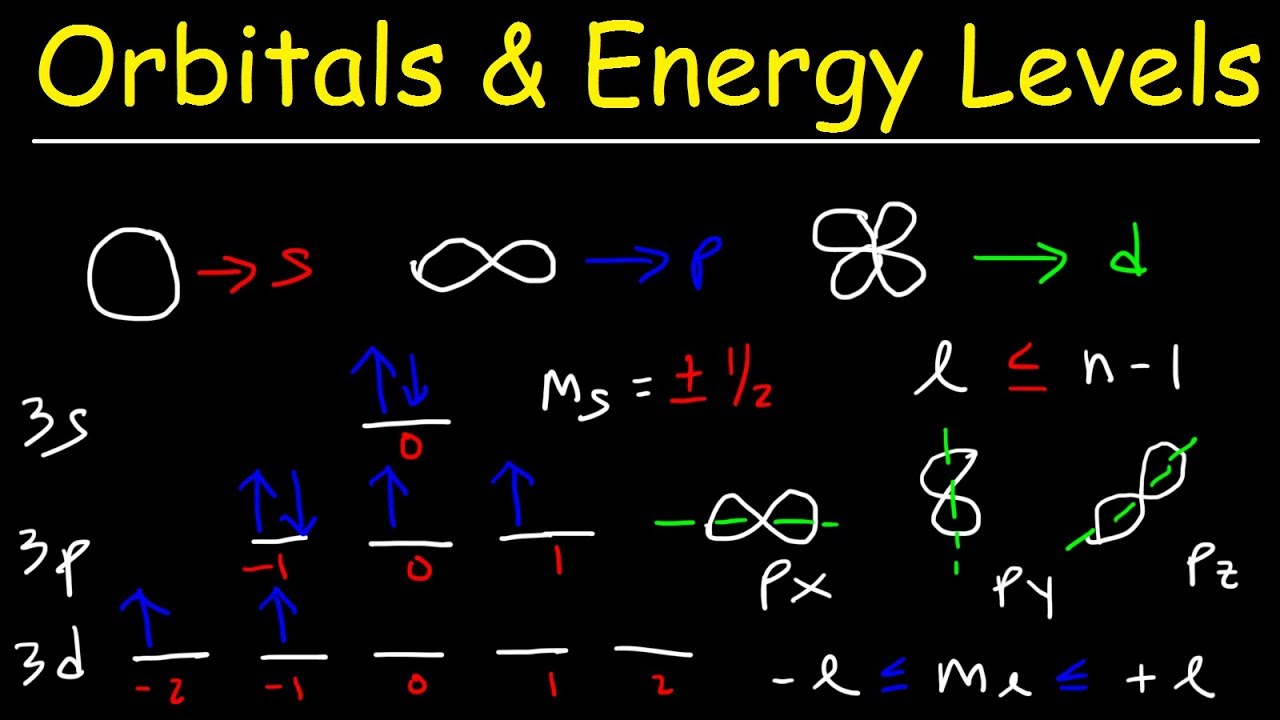
Spdf Orbitals : Parsing Spdf Orbital Hybridization And Simple Bonding

2.2: Electron Configurations - Chemistry LibreTexts

Electron Configurations | CK-12 Foundation
